Charged droplets occur everywhere in the world. They are created by the oceans (known as see spray aerosols), near waterfalls and in thunderstorm clouds. Such droplets are expected to play significant role in environmental processes. Similar droplets are also created in electrospray ionization (ESI) source.
Mari Ojakivi joined our group three years ago to conduct her bachelor thesis with us. We were then very strongly interested in the way different additives influence ionization efficiencies in ESI. So she started studying how different acids, salts and bases influence the ionization of some amines in charged water droplets. Soon, some extremely interesting results were revealed that allowed us to make much wider conclusions about charged droplets.
We were able to pinpoint, that protonation of the amines is strongly dependent on the type of additives present in the droplets and is virtually independent of the pH of the solution used for “preparing” the droplets. In “normal” solutions the protonation determined solely by the pH of the solution. This led us to conclude that some of the additives change something about the droplets that other additives do not affect. It turned out, that the factor determining the protonation is the cation present near the surface of the charged droplets, as the protonation mostly takes place on the surface. Cations, such as hydronium ion (droplet A in the picture), that are strong acids protonate the compounds, while weak acids, such as ammonium cation (droplet B in the picture), do not. If both types of cations are present in the solution, the protonation is determined by the ion that has higher affinity for the droplets surface. We were also able to find support for our model from the molecular dynamics simulations carried out in Prof. Konermann’s group.
Why is the protonation in charged droplets at all important? Protonation is one of the fundamental properties of compounds; it may catalyze reactions, break up or induce complexation, change conformation of the macromolecules, etc. Therefore, it can be assumed, that the reactions and processes taking place in charged droplets also depend on the protonation.
Modifying the Acidity of Charged Droplets. Mari Ojakivi, Jaanus Liigand, Dr. Anneli Kruve. Chemistry Select doi.org/10.1002/slct.201702269
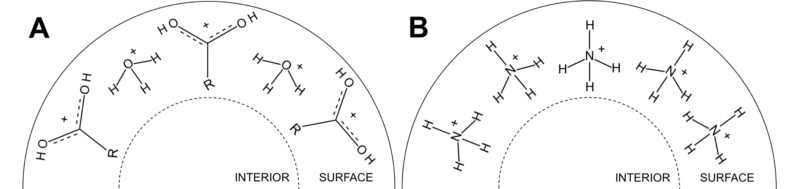
Illustration of droplets surface for droplets that do not contain ammonium anion (A) and that do contain ammonium ions (B). The latter significantly decreases the protonation of the compounds in the droplet. Picture by M. and K. Ojakivi.