Recently my first single-authored paper became public. It concerns the solvent effect on electrospray ionization efficiency and matrix effect. As we have talked about ionization efficiency and its solvent effects also previously, let’s focus on matrix effects this time.
As LC/ESI/MS is the tool for various fields in routine analyses (quality control over pesticides but also mycotoxins in the food we eat, drug monitoring in patient’s body, etc) to solving highly demanding scientific questions (finding biomarkers, decoding nervous signalling, etc). However, one thing haunting quantitation in these analyses is the matrix effect. The influence of sample compounds (called matrix compounds; for example the flavor compounds in tomato) on the ionization of our target analyte (e.g. the monitored mycotoxin in tomato). This effect may both give results that are either higher or lower than the actual content. Though underestimation is more common. Meaning that the mycotoxin may actually exceed the allowed limits, but matrix effect may hinder our ability to detect this. This makes matrix effect very important and the need to remove its origin becomes obvious.
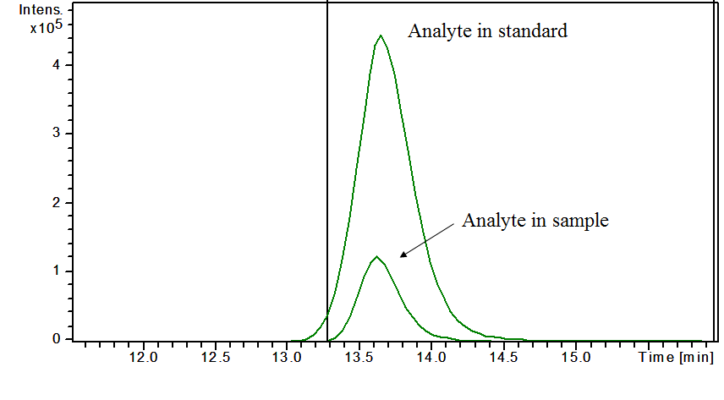
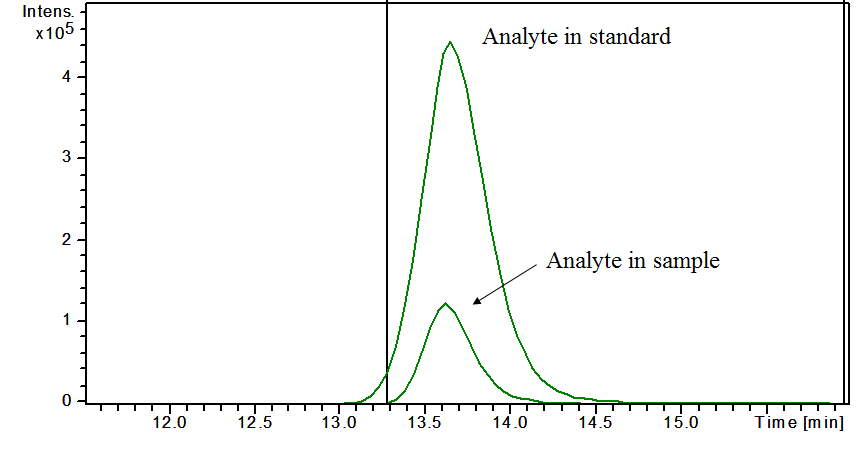
This is how matrix effect looks on a chromatogram.
Though, since I started interacting with this field in the beginning of my Master‘s thesis, one thing has definitely improved: the awareness of the presence of matrix effect. Still much unclarity exists in the fundamental level. There have been numerous mechanisms proposed to the origin of matrix effect, however some of them are contradictory or have not been reproduced latter. However, there is one general mathematical model proposed by Christie Enke in 1997, which states that matrix effect is caused by the fact that two compounds compete for the surface charge in the ESI droplets and that the extent of matrix effect is related to the magnitude of these affinities. However, it is unknown of what these affinities depend on. In one of my previous works I discovered that this affinity is well correlated with ionization efficiencies for ionisable compounds1.
During investigating ionization efficiencies dependents on solvent composition3 naturally the question arouse: if compounds‘ ionization efficiencies depend on the solvent composition why shouldn’t the matrix effect do2? And it does! It was observed that solvent compositions that provide higher ionization efficiencies produce more matrix effect. Higher ionization efficiencies are usually considered beneficial because they allow analysing smaller analyte quantities (limit of quantitation, LoQ) and increase sensitivity. So if you tune your method for lower LOD-s it will automatically be less robust for matrix effects. It turns out that in ESI/MS you have to balance between low LoD-s and little matrix effect, while choosing your solvent.
With this study I was able to uncover the true dual nature of the processes occurring in the charged nanodroplets in ESI. Though the practical conclusions are not according to every analyst dreams4 it effectively demonstrates the dual side of the processes occurring in micro compartments such as nanodroplets. Therefore, I find that these nanodroplets are capable of much more and we shall hear about these again.
1 It is known that also compounds that do not give ESI/MS signal also produce matrix effect; however, this process is expected to follow a different mechanism.
2 Previously different solvent conditions have been also used to reduce or eliminate matrix effect, but with the aim of providing better separation of the analyte and matrix components.
3 Solvent with higher organic content tends to yield higher ionization efficiencies.
4 It would be best to have lowest LoD-s, no matrix effect, high sensitivity, etc all at once.