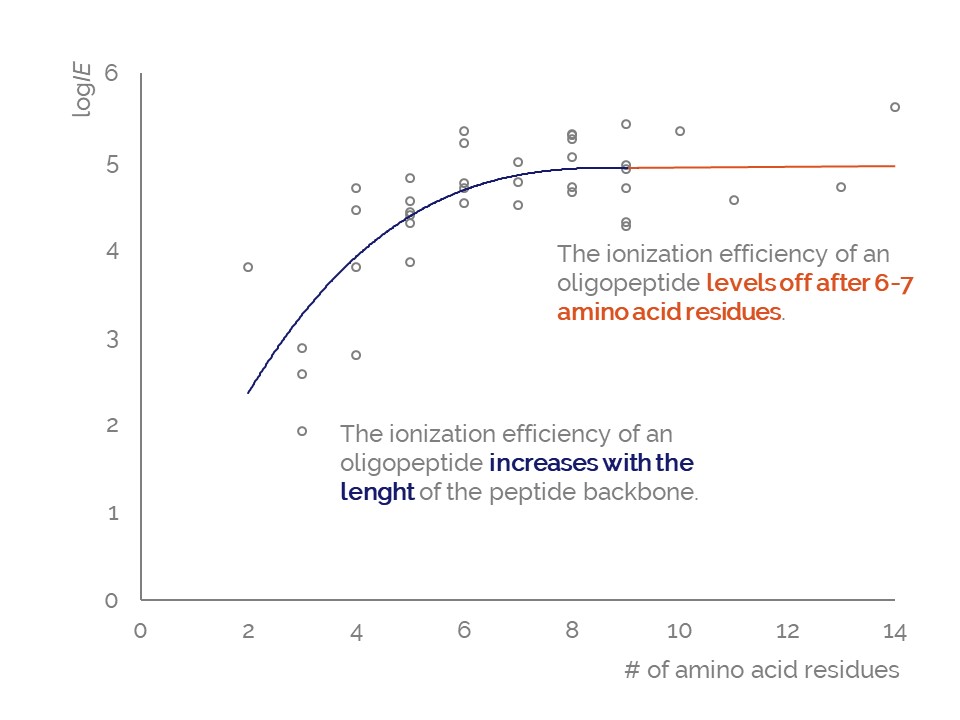
Ionization efficiency of oligopeptides does depend on the chemical composition of the peptide. Longer oligopeptides generally obey higher ionization efficiency; however, for peptides containing more than seven amino acid the ionization efficiency values are relatively similar. Also, the ionization efficiency values of the amino acids can be used to estimate the ionization efficiency of a specific peptide.
Over the last couple of years, we have investigated the ionization efficiency for small singly charged molecules in electrospray. In the conferences, we are often asked what do we know about the ionization efficiency of larger and multiply charged biomolecules. It is known that oligopeptides ionize via chain ejection model (CEM) in ESI while small molecules ionize through ion evaporation model (IEM). Therefore, the findings from studies of small molecules are not directly applicable for oligopeptides.
Here we investigated the ionization efficiency of 38 oligopeptides with 2 to 14 amino acid residues. The longest oligopeptide had highest ionization efficiency (logIE = 5.61) and one of the smallest and most hydrophilic oligopeptides (Gly-Gly-Gly-NH2) had the lowest ionization efficiency (logIE = 1.92). This means that the sensitivity difference between the best and the worst ionizing compound is ca 5000 times. It also became obvious that from length 5-6 amino acids onwards the ionization efficiency values level off: the increase in oligopeptide length does not increase ionization efficiency significantly.
The similarities observed for the ionization behaviour of amino acids and oligopeptides suggest that similar physicochemical parameters are significant for describing their ionization efficiencies. Therefore, we tested predicting ionization efficiency of an oligopeptide based on the ionization efficiency of the amino acids it consists of. The predicted logIE values were in a good correlation with the measured values (R2 = 0.70). You can read more from Journal of Mass Spectrometry.