Today I would like to introduce you to two books that I find very valuable and that can be freely downloaded from the internet. The “Five keys to successful LC methods” and “Controlling selectivity in reversed-phase LC” are collections of articles published in LC/GC in the section “LC Troubleshooting” by John W. Dolan. It’s useful whether you are a beginner learning on you own or a specialist working with LC every day. I can assure that even the latter will find something refreshing in these writings. And of course I have to admit I am a big fan of John W. Dolan. His columns in LC/GC are both a good study material but often also a fascinating detective story for an analytical chemists (eg this one here: http://www.chromatographyonline.com/calibration-problems-case-study-1).
Both books are freely available from the LC/GC homepage (http://www.chromatographyonline.com/lcgc-ebooks).
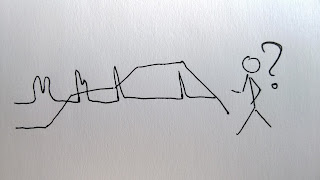
Here I would also like to come back to one of J.W. Doles thoughts that has really influenced me. I think everyone working with more than one compound or with matrix containing various stuff, have found themselves stuck with figuring out the best gradient to fit the resolution and speed desired. And of course this perfect gradient needs to be figured out fast to not waste any precious analyses time. Well, one really simple but hard to do thing to keep in mind is: First things first. Don’t mess with the middle part of your LC gradient before you have a needed separation for the early eluting peaks. Firstly, this is really pointless – changes in the beginning will very likely influence resolution in the middle part as well. Secondly, it just waists extra time – you will most probably have to do this once again after you have made the necessary changes in the beginning of the gradient. I know from my own experiences that it is hard to stick to this rule but it sure pays off! I would even extend this not only to LC gradient but also to other fields, eg sample prep – your solution composition most probably is influenced by the dissolving, pH regulation, etc steps you carry out in the beginning of the sample prep. Therefore before you start working out your SPE you should know in what form (including solvent etc your sample is by this stage. Of course, here this is even harder, as if SPE is not working at all (eg analyte flushes through the cartridge during sample introduction) it is also hard to optimize earlier steps (unless you can make injections without SPE).